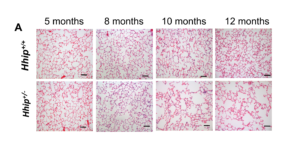
 Though GWAS is powerful to pinpoint novel disease candidate genes, the importance of GWAS genes in vivo has been questionable. Our findings in Hhip+/-mouse line, a suitable model to recapitulate human subjects carrying the COPD risk allele at the HHIP (Hedgehog Interacting Protein) locus (which have similar levels of HHIP reduction), provided supporting evidence for the biological impacts of GWAS variants. We have demonstrated association of the COPD risk allele with reduced HHIP expression through chromatin long-range interactions, and an ~32% reduction in HHIP mRNA expression in human COPD lung samples. Moreover, developmentally normal Hhip heterozygous mice (Hhip+/-) demonstrated a ~33% reduction of HHIP in lung tissues and demonstrated increased susceptibility to CS-induced emphysema, recapitulating human genetic discoveries at the HHIP locus. Hhip+/-micedeveloped increased susceptibility to age- and smoke-induced emphysema and lung functional decline, providing in vivo evidence that Hhip locus is associated with lung function in both COPD patients and general non-smoker populations. Furthermore, Hhip+/-mouse model also provides a very strong translational potentiation for mechanistic studies that will lead to novel drugs that will be tested in this murine model and eventually to smokers carrying risk genetic factors for COPD at the HHIP locus.
Though GWAS is powerful to pinpoint novel disease candidate genes, the importance of GWAS genes in vivo has been questionable. Our findings in Hhip+/-mouse line, a suitable model to recapitulate human subjects carrying the COPD risk allele at the HHIP (Hedgehog Interacting Protein) locus (which have similar levels of HHIP reduction), provided supporting evidence for the biological impacts of GWAS variants. We have demonstrated association of the COPD risk allele with reduced HHIP expression through chromatin long-range interactions, and an ~32% reduction in HHIP mRNA expression in human COPD lung samples. Moreover, developmentally normal Hhip heterozygous mice (Hhip+/-) demonstrated a ~33% reduction of HHIP in lung tissues and demonstrated increased susceptibility to CS-induced emphysema, recapitulating human genetic discoveries at the HHIP locus. Hhip+/-micedeveloped increased susceptibility to age- and smoke-induced emphysema and lung functional decline, providing in vivo evidence that Hhip locus is associated with lung function in both COPD patients and general non-smoker populations. Furthermore, Hhip+/-mouse model also provides a very strong translational potentiation for mechanistic studies that will lead to novel drugs that will be tested in this murine model and eventually to smokers carrying risk genetic factors for COPD at the HHIP locus.